104146-53-4
| Name | Cefditoren Acid Sodium Salt |
|---|---|
| Synonyms |
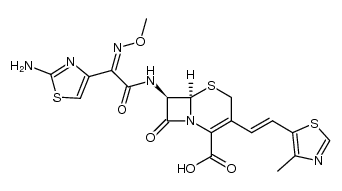
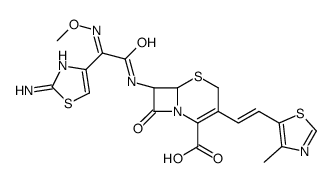
sodium (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyimino-acetyl]amino]-3-[(E)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
ME-1206 (6R-(3(Z),6alpha,7beta(Z)))-7-(((2-Amino-4-thiazolyl)(methoxyimino)acetyl)amino)-3-(2-(4-methyl-5-thiazolyl)ethenyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monosodium salt Cefditoren sodium [6R-[3(Z),6a,7b(Z)]]-7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-[2-(4-methyl-5-thiazolyl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-carboxylic acid sodium salt sodium (6R,7R)-7-[2-amino-4-thiazolyl[(methoxyimino)acetyl]amino]-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-3-cephem-4-carboxylate (6R,7R)-7-[(Z)-2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetylamino]-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]octa-2-ene-2-carboxylic acid sodium salt Cefditoren acid,sodium salt 7-<(Z)-2-(2-Aminothiazol-4-yl)-2-methoxyiminoacetamido>-3(Z)-(4-methylthiazol-5-yl)vinyl-3-cephem-4-carboxylic acid sodium salt |
| Description | Cefditoren sodium (ME 1206) is a broad-spectrum, third-generation, oral cephalosporin antibacterial with enhanced stability against many common β lactamases. Cefditoren sodium has activity against Gram-negative organisms and Gram-positive organisms. Cefditoren sodium can be used in the research of infection diseases such as acute exacerbations of chronic bronchitis, community-acquired pneumonia (CAP), streptococcal pharyngitis/tonsillitis, or uncomplicated skin and skin structure infections[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Cefditoren (sodium) shows good activity against pneumoniae, S. pyogenes, Staphylococcus aureus, H. influenzae and H. parainfluenzae, M. catarrhalis[1]. Organism MIC50 (mg/L) MIC90 (mg/L) susceptible (%) Gram-positive organisms Streptococcus pneumoniae (untyped)a 0.015-0.25 0.12-1 PS ≤0.008-0.03 0.015-0.25 99-100 PI 0.06-0.5 0.25-0.5 94-100 PR 0.25-1 0.5-2 7-100 S. pyogenes ≤0.008-0.03 0.015-0.03 100 Staphylococcus aureus (MS) 0.12-0.5 0.5-1 50-100 Gram-negative organisms Haemophilus ≤0.008-≤0.03 0.015-0.13 influenzae (untyped)b β-Lactamase + ≤0.008-0.03 0.015-0.06 100 β-Lactamase - ≤0.008-0.03 0.015-0.06 100 H. parainfluenzaeb ≤0.03 0.06 Moraxella 0.03-0.5 0.25-1 96 catarrhalis (untyped)b β-Lactamase + ≤0.03-0.25 0.12-0.5 100 β-Lactamase - ≤0.008-0.03 0.015-0.06 100 a Including PS, PI and PR strains. b Including both β-lactamase-positive and -negative strains. MIC50/90=mean minimum inhibitory concentrations required to inhibit the growth of 50% or 90% of bacterial strains; MS=susceptible to meticillin; PI=intermediate susceptibility to penicillin; PR=resistant to penicillin; PS=susceptible to penicillin. |
| In Vivo | Pharmacokinetics in mice[1]: Cefditoren (mg/kg) C0 (µg/ml) t1/2 (h) AUClast (µg× h/ml) Tlast (h) 6.25 53.0 0.9 30.4 6 12.5 168.4 1.1 64.1 8 25 232.5 0.9 101.3 8 50 290.6 1.1 124.4 8 |
| References |
| Molecular Formula | C19H17N6NaO5S3 |
|---|---|
| Molecular Weight | 528.56000 |
| Exact Mass | 528.03200 |
| PSA | 244.71000 |
| LogP | 0.86800 |
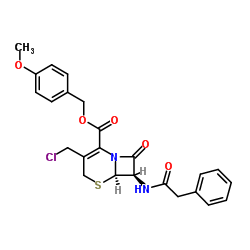
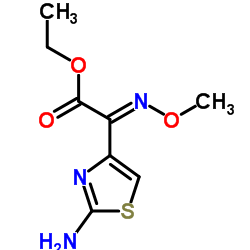
| Precursor 7 | |
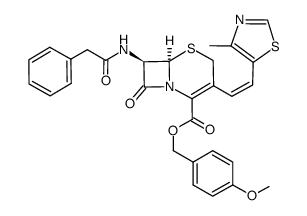
|---|---|
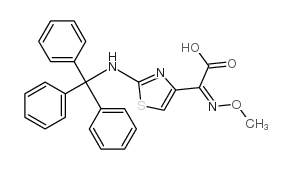
| DownStream 1 | |