90-33-5
| Name | 4-Methylumbelliferone |
|---|---|
| Synonyms |
LM 94
7-hydroxy-4-methyl-coumarin BMU CantabbY Medilla 7-hydroxy-4-methyl-chromen-2-one 4-Methylumbelliferone EINECS 201-986-7 Crodimon 4-methyl-7-hydroxy coumarin Bilcolic Himecol MFCD00006866 7-hydroxy-4-methyl-2H-chromen-2-one Eurogale 7-Hydroxy-4-methylcoumarin |
| Description | 4-Methylumbelliferone is a hyaluronic acid biosynthesis inhibitor with antitumoral and antimetastatic effects. |
|---|---|
| Related Catalog | |
| Target |
hyaluronic acid[1] |
| In Vitro | 4-Methylumbelliferone (4-MU) affects several key steps of angiogenesis, including endothelial cell proliferation, adhesion, tube formation, and extracellular matrix remodeling. Half-maximal inhibitory concentrations (IC50) values in the proliferation assay are 0.65±0.04 and 0.37±0.03 mM for HMEC and RF-24 endothelial cells, respectively. 4-Methylumbelliferone (2 mM) treatment for 24 h induces apoptosis in 13% of HMEC and 5% of RF-24 cells. The number of adherent endothelial cells decreases by >20% after 24 h of treatment with 1 mM 4-Methylumbelliferone. Minimal inhibitory concentrations in the tube formation assay are 2 and 0.5 mM 4-Methylumbelliferone for HMEC and RF-24, respectively. Matrix metalloproteinase-2 expression is differentially altered upon 4-Methylumbelliferone treatment in both tested endothelial cell lines[1]. |
| Cell Assay | The MTT dye reduction assay in 96-well microplates is used. The assay is dependent on the reduction of MTT by mitochondrial dehydrogenases of viable cells to a blue formazan product, which can be measured spectrophotometrically. Endothelial cells (2.5×103 cells in a total volume of 100 μL of complete medium) are incubated in each well with serial dilutions of 4-Methylumbelliferone (4-MU) (0, 0.5, 1, 1.5 and 2 mM). After 3 days of incubation in the dark (37°C, 5% CO2 in a humid atmosphere), 10 μL of MTT (5 mg/mL in PBS) is added to each well, and the plate is incubated for further a 4 h (37°C). The formazan is dissolved in 150 μL of 0.04 N HCl-2 propanol, and samples are spectrophotometrically measured at 550 nm. All determinations are carried out in quadruplicate, and at least three independent experiments are carried out. IC50 values are calculated as those concentrations of compound yielding 50% cell survival, taking the values obtained for control as 100%[1]. |
| References |
| Density | 1.319 g/cm3 |
|---|---|
| Boiling Point | 377.4ºC at 760 mmHg |
| Melting Point | 188.5-190 °C(lit.) |
| Molecular Formula | C10H8O3 |
| Molecular Weight | 176.16900 |
| Flash Point | 174.5ºC |
| Exact Mass | 176.04700 |
| PSA | 50.44000 |
| LogP | 1.80700 |
| Storage condition | -20°C Freezer |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | slightly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | GN7000000 |
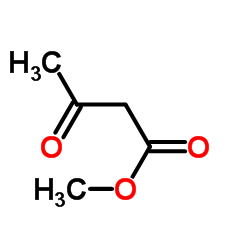
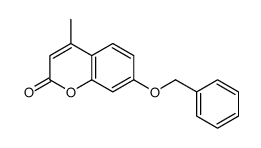
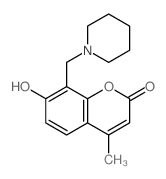
| Precursor 9 | |
|---|---|
| DownStream 10 | |




![4-methyl-[1]benzofuro[3,2-g]chromen-2-one structure](https://image.chemsrc.com/caspic/460/105290-13-9.png)