120615-25-0
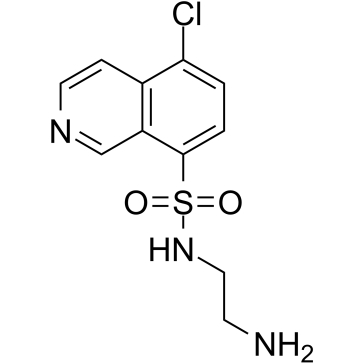
| Name | N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide |
|---|---|
| Synonyms |
N-(2-Aminoethyl)-5-chloroisoquinoline-8-sulphonamide
CKI-7 2csn CKI 8-Isoquinolinesulfonamide,N-(2-aminoethyl)-5-chloro |
| Description | CKI-7 is a potent and ATP-competitive casein kinase 1 (CK1) inhibitor with an IC50 of 6 μM and a Ki of 8.5 μM. CKI-7 is a selective Cdc7 kinase inhibitor. CKI-7 also inhibits SGK, ribosomal S6 kinase-1 (S6K1) and mitogen- and stress-activated protein kinase-1 (MSK1). CKI-7 has a much weaker effect on casein kinase II and other protein kinases[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
CK1:6 μM (IC50) CK1:8.5 μM (Ki) Cdc7 SGK S6K1 MSK1 |
| In Vitro | CKI-7 (0.1-10 μM; 5 days; ES cells) treatment significantly increases the expression of the early neuroectodermal marker Sox1 and the number of cells positive for the neural markers nestin and βIII-tubulin, in a concentration-dependent manner[1]. CKI-7 (5 μM; 5 days; ES cells) treatment suppresses SFEB-induced β-catenin stabilization on day 5, indicating that CKI-7 inhibits Wnt signaling[1]. RT-PCR[1] Cell Line: Mouse ES cells Concentration: 0.1-10 μM Incubation Time: 5 days Result: Significantly increased the expression of the early neuroectodermal marker Sox1 and the number of cells positive for the neural markers nestin and βIII-tubulin, in a concentration-dependent manner. Western Blot Analysis[1] Cell Line: Mouse ES cells Concentration: 5 μM Incubation Time: 5 days Result: Suppressed SFEB-induced β-catenin stabilization on day 5. |
| In Vivo | In vivo dose-dependent anti-tumor activity of CKI-7 is demonstrated in a SCID-Beige mouse systemic tumor model utilzing a recently isolated Philadelphia chromosome positive acute lymphoblastic leukemia cell line. Standard cell cycle synchronization studies established that exposure to CKI-7 results in cell cycle dependent caspase 3 activation and apoptotic cell death[2]. |
| References |
| Density | 1.432g/cm3 |
|---|---|
| Boiling Point | 499.7ºC at 760mmHg |
| Melting Point | 188-190ºC |
| Molecular Formula | C11H12ClN3O2S |
| Molecular Weight | 285.75000 |
| Flash Point | 256ºC |
| Exact Mass | 285.03400 |
| PSA | 93.46000 |
| LogP | 3.29720 |
| Vapour Pressure | 4.04E-10mmHg at 25°C |
| Index of Refraction | 1.644 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |