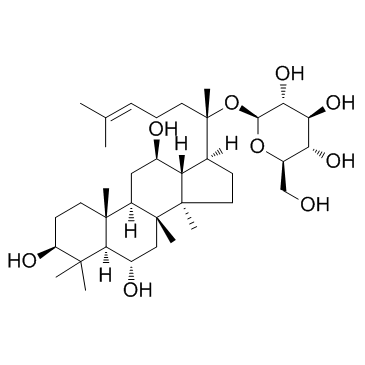
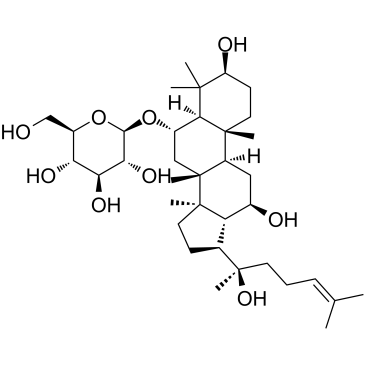
52286-59-6
| Name | ginsenoside Re |
|---|---|
| Synonyms |
β-D-Glucopyranoside, (3β,6α,12β)-20-(β-D-glucopyranosyloxy)-3,12-dihydroxydammar-24-en-6-yl 2-O-(6-deoxy-α-L-mannopyranosyl)-
chikusetsusaponinivc Ginsenoside Re Panaxoside (2S,3R,4S,5S,6R)-2-({(2S)-2-[(3S,5R,6S,8R,9R,10R,12R,13R,14R,17S)-6-{[(2R,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-2-yl]oxy}-3,12-dihydroxy-4,4,8,10,14-pentamethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methyl-5-hepten-2-yl}oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (3β,6α,12β)-20-(β-D-Glucopyranosyloxy)-3,12-dihydroxydammar-24-en-6-yl 2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside gingenoside Re ginsenoside-Re standard (3β,6α,12β)-20-(β-D-Glucopyranosyloxy)-3,12-dihydroxydammar-24-en-6-yl-2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside ginsenoside-Re GINSENOSIDE GINSENOSIDE-RE EINECS 257-814-6 ginsenosideb2 (3β,6α,12β)-6-{[2-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy}-3,12-dihydroxydammar-24-en-20-yl β-D-glucopyranoside MFCD00133369 (2S,3R,4S,5S,6R)-2-({(2S)-2-[(3S,5R,6S,8R,9R,10R,12R,13R,14R,17S)-6-{[(2R,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxyméthyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-méthyltétrahydro-2H-pyran-2-yl]oxy}tétrahydro-2H-pyran-2-yl]oxy}-3,12-dihydroxy-4,4,8,10,14-pentaméthylhexadécahydro-1H-cyclopenta[a]phénanthrén-17-yl]-6-méthyl-5-heptèn-2-yl}oxy)-6-(hydroxyméthyl)tétrahydro-2H-pyran-3,4,5-triol β-D-Glucopyranoside, (3β,6α,12β)-6-[[2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-3,12-dihydroxydammar-24-en-20-yl |
| Description | Ginsenoside Re is an extract from Panax notoginseng. Ginsenoside Re decreases the β-amyloid protein (Aβ). Ginsenoside Re plays a role in antiinflammation through inhibition of JNK and NF-κB. |
|---|---|
| Related Catalog | |
| Target |
Aβ1-40 Aβ1-42 NF-κB JNK |
| In Vitro | Ginsenoside Re is a well-known traditional Chinese medicine, which decreases the β-site amyloid precursor protein cleaving enzyme 1 (BACE1) mRNA and protein levels and inhibits BACE1 activity in the N2a/APP695 cells. Ginsenoside Re also significantly increases the PPARγ protein and mRNA levels.To prevent Ginsenoside Re from having a cytotoxic effect on the N2a/APP695 cells, the cell viability is first determined by the MTT assay. The N2a/WT and N2a/APP695 cells are treated with increasing concentrations of Ginsenoside Re (0-200 µM) for 24 h. Ginsenoside Re concentrations under 100 µM do not affect the viability of the N2a/WT and N2a/APP695 cells, whereas the 150 µM Ginsenoside Re concentration markedly decreases the survival rate of the N2a/WT and N2a/APP695 cells. Incubation with Ginsenoside Re at a 200 µM concentration for 24 h reduces the viability of the N2a/WT and N2a/APP695 cells by 15.58% and 26.82%, respectively. These data indicate that Ginsenoside Re treatment within the range of 0-100 µM for 24 h is safe for the N2a/WT and N2a/APP695 cells (P>0.05)[1]. |
| In Vivo | Ginsenoside Re reduces insulin resistance in 3T3-L1 adipocytes and high-fat diet (HFD) rats through inhibition of JNK and NF-κB activation[2]. Intraperitoneal injection of lipopolysaccharide (LPS) at a dose of 20 mg/kg is lethal to mice, and 70% to 80% of the mice die within 60 h. However, pretreatment of the mice with Rg1 or Ginsenoside Re increases their survival rates in a dose-dependent manner. With the doses of Rg1 or Ginsenoside Re increase from 2.5 to 5 mg/kg, the survival rate is elevated from 60% to 90% (Rg1) or from 30% to 40% (Ginsenoside Re). All the mice administered Rg1 at a minimal dose of 10 mg/kg are protected from death compared to 80% survival of mice treated with an equal dose of Ginsenoside Re. To protect all the mice, 20 mg/kg Ginsenoside Re is needed. To investigate the anti-inflammatory potential of Rg1 and Ginsenoside Re, 1 mg/kg Rg1 or Ginsenoside Re is injected into rats and then challenged the animals with LPS. The injection procedure itself causes a transient stress-induced increase in body temperature of ~1.2°C in each group. Thereafter, LPS-challenged rats without pretreatment develope a robust biphasic fever, with the first peak reaching ~1.5°C at 2 h and the second peak reaching 1.8°C at 4 h. In contrast, the temperature changes for the Rg1-, Ginsenoside Re-, and TAK-242-treated groups are only 0.9, 1.2, and 0.8°C at 2 h and 1.3, 1.4, and 1.0°C at 4 h, respectively. Pretreatment with Rg1, Ginsenoside Re, or TAK-242 significantly attenuates LPS-induced alterations in body temperature[3]. |
| Cell Assay | To explore the cytotoxic effect of Ginsenoside Re on N2a/APP695 cells, cell proliferation is assessed using the MTT assay. The cells are treated with increasing doses of Ginsenoside Re (0, 25, 50, 100, 150, and 200 µM) for 24 h. The MTT assay is performed after the treatments. The cells are incubated for 4 h at 37 °C with 0.5 mg/mL of MTT dissolved in fresh complete medium. The dark blue formazan crystals are dissolved in DMSO, and the absorbance is measured on a microplate reader using a reference wavelength of 630 nm and a test wavelength of 490 nm. The data are expressed as the mean percentages of viable cells versus the control[1]. |
| Animal Admin | Mice[3] To examine the prophylactic effect of Rg1 and Ginsenoside Re on LPS-induced lethality, 6- to 8-week-old BALB/c mice are randomly assigned to 7 groups with 10 mice in each group. The mice are either left untreated or subcutaneously injected with 2.5, 5, 10, or 20 mg/kg Rg1, 20 mg/kg Ginsenoside Re, or 5 mg/kg TAK-242 3 times at 30-min intervals and then challenged with LPS (20 mg/kg) 15 min later. To test the therapeutic effect of Rg1 and Ginsenoside Re on LPS-induced lethality, BALB/c mice are assigned to 5 groups with 10 mice in each group. The mice are injected intraperitoneally (i.p.) with 20 mg/kg LPS. Fifteen minutes later, mice are subcutaneously injected with 10 mg/kg Rg1, 20 mg/kg Ginsenoside Re, or 5 mg/kg TAK-242 3 times at 30-min intervals or left untreated. Survival rates are recorded for 60 h. Rats[3] Sprague Dawley rats are intravenously administered saline solution, 1 mg of Rg1/kg body weight (1 mg/kg Rg1), 1 mg/kg Ginsenoside Re, or 1 mg/kg TAK-242. Fifteen minutes later, rats are challenged with 2.5 μg/kg LPS. Body temperature is measured before and after drug administration. Anticoagulated blood samples with EDTA are collected for white blood cell (WBC) counts at indicated time points using a ProCyte Dx automatic blood cell analyzer. Additional blood samples are collected at 4 h post-drug injection and used for preparation of serum to analyze proinflammatory mediators. The proinflammatory cytokine responses are detected by Western blot assay. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 1011.8±65.0 °C at 760 mmHg |
| Melting Point | 202 °C(dec.) |
| Molecular Formula | C48H82O18 |
| Molecular Weight | 947.154 |
| Flash Point | 565.7±34.3 °C |
| Exact Mass | 946.550110 |
| PSA | 298.14000 |
| LogP | 4.75 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.611 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LY9536700 |
|
~% 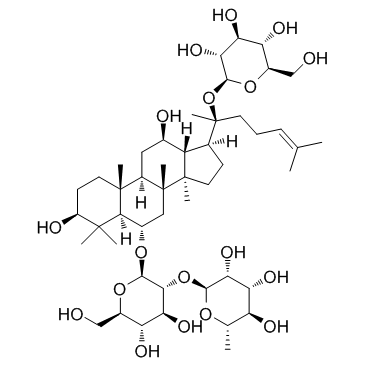
52286-59-6 |
| Literature: Morita; Kong; But; et al. Chemical and Pharmaceutical Bulletin, 1986 , vol. 34, # 10 p. 4368 - 4372 |
| Precursor 0 | |
|---|---|
| DownStream 5 | |