Diosmetin
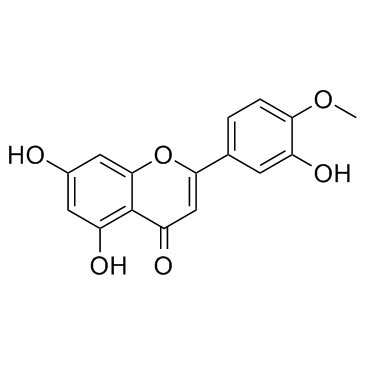
Diosmetin structure
|
Common Name | Diosmetin | ||
|---|---|---|---|---|
| CAS Number | 520-34-3 | Molecular Weight | 300.263 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 576.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H12O6 | Melting Point | 256-258ºC | |
| MSDS | Chinese USA | Flash Point | 220.3±23.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of DiosmetinDiosmetin is a natural flavonoid which inhibits human CYP1A enzyme activity with an IC50 of 40 μM in HepG2 cell. |
| Name | diosmetin |
|---|---|
| Synonym | More Synonyms |
| Description | Diosmetin is a natural flavonoid which inhibits human CYP1A enzyme activity with an IC50 of 40 μM in HepG2 cell. |
|---|---|
| Related Catalog | |
| Target |
IC50: 40 μM (Others, HepG2 cell)[1] |
| In Vitro | Diosmetin inhibits cell proliferation in HepG2 cells in a concentration-dependent manner. Untreated HepG2 cells grow well and are observed to have with normal skeletons, whereas cells treated with diosmetin are distorted and a number of them become round and floating[1]. |
| In Vivo | Pretreatment with diosmetin significantly reduces serum levels of amylase and lipase; the histological injury; the secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6; myeloperoxidase (MPO) activity, trypsinogen activation peptide (TAP) level, the expression of inducible nitric oxide synthase (iNOS); and the nuclear factor (NF)-κB activation in cerulein-induced acute pancreatitis[2]. |
| Cell Assay | Diosmetin is dissolved in DMSO which is maintained at a constant concentration in control samples (2%). HepG2 cells are maintained in a humidified atmosphere of 5% CO2 at 37°C, and cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin. HepG2 cell density is adjusted to 2×104 cells/100 μL, and the cells are seeded into 96-well plates and placed in an incubator overnight (37°C in 5% CO2) to allow for attachment and recovery. MTT analyses are performed. Briefly, cells are pretreated with 5, 10, 15 and 20 μg/mL diosmetin for 24 h. A total of 20 μL MTT solution (5 mg/mL in PBS) solution is transferred to each well to yield a final 120 μL/well and to separate wells a total of 10 μL CCK8 (5 mg/mL in PBS) is transferred. The plates are incubated for 4 h at 37°C in 5% CO2 and the absorbance is recorded at wavelengths of 595 nm and 450 nm, respectively. The half maximal inhibitory concentration (IC50) of diosmetin is calculated[1]. |
| Animal Admin | Experimental acute pancreatitis is induced in mice by seven intraperitoneal injection of cerulein (50 μg/kg) at hourly intervals. Diosmetin (100 mg/kg) or vehicle is pretreated 2 h before the first cerulein injection. After 6 h, 9 h, 12 h of the first cerulein injection, the severity of acute pancreatitis is evaluated biochemically and morphologically[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 576.7±50.0 °C at 760 mmHg |
| Melting Point | 256-258ºC |
| Molecular Formula | C16H12O6 |
| Molecular Weight | 300.263 |
| Flash Point | 220.3±23.6 °C |
| Exact Mass | 300.063385 |
| PSA | 100.13000 |
| LogP | 3.10 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.697 |
| Storage condition | -20?C Freezer |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2942000000 |
| Precursor 9 | |
|---|---|
| DownStream 6 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Antiviral effect of methylated flavonol isorhamnetin against influenza.
PLoS ONE 10(3) , e0121610, (2015) Influenza is an infectious respiratory disease with frequent seasonal epidemics that causes a high rate of mortality and morbidity in humans, poultry, and animals. Influenza is a serious economic conc... |
|
|
Differential metabolomic analysis of the potential antiproliferative mechanism of olive leaf extract on the JIMT-1 breast cancer cell line.
J. Pharm. Biomed. Anal. 105 , 156-62, (2015) A new differential metabolomic approach has been developed to identify the phenolic cellular metabolites derived from breast cancer cells treated with a supercritical fluid extracted (SFE) olive leaf ... |
|
|
Flavonoids are inhibitors of human organic anion transporter 1 (OAT1)-mediated transport.
Drug Metab. Dispos. 42(9) , 1357-66, (2014) Organic anion transporter 1 (OAT1) has been reported to be involved in the nephrotoxicity of many anionic xenobiotics. As current clinically used OAT1 inhibitors are often associated with safety issue... |
| Vitamin P |
| 3',5,7-dihydroxy-4'-methoxyflavone |
| Cyanidenon-4'-methyl Ether 1479 |
| 3,3',5,7-tetrahydroxy-4'-methoxyflavone |
| 4'-Methylluteolin |
| 5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one |
| 5,7,3'-Trihydroxy-4'-methoxyflavone |
| MFCD00017425 |
| 3',5,7-trihydroxy-4'-methoxy-2-phenylchromone |
| 5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one |
| Luteolin 4'-methyl ether |
| 3',5,7-TRIHYDROXY-4'METHOXYFLAVONE |
| Diosmetin |
| 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)- |
| Luteolin-4'-methyl Ether |
| 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chromen-4-one |
| EINECS 208-291-8 |
| Salinigricoflavonol |
 CAS#:520-33-2
CAS#:520-33-2 CAS#:3162-05-8
CAS#:3162-05-8 CAS#:748-62-9
CAS#:748-62-9 CAS#:520-27-4
CAS#:520-27-4 CAS#:20126-59-4
CAS#:20126-59-4![[(2R)-7-acetyloxy-2-(3-acetyloxy-4-methoxy-phenyl)-4-oxo-chroman-5-yl] acetate Structure](https://image.chemsrc.com/caspic/298/6274-73-3.png) CAS#:6274-73-3
CAS#:6274-73-3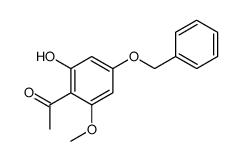 CAS#:39548-89-5
CAS#:39548-89-5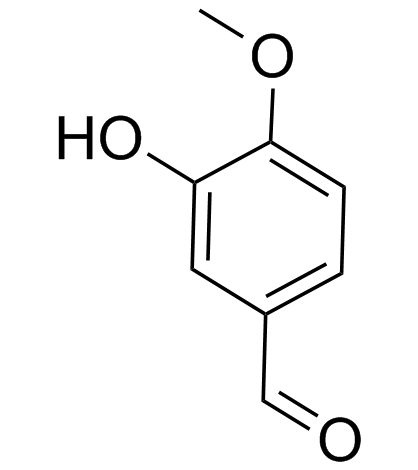 CAS#:621-59-0
CAS#:621-59-0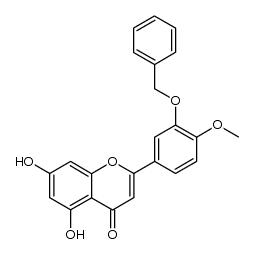 CAS#:102593-81-7
CAS#:102593-81-7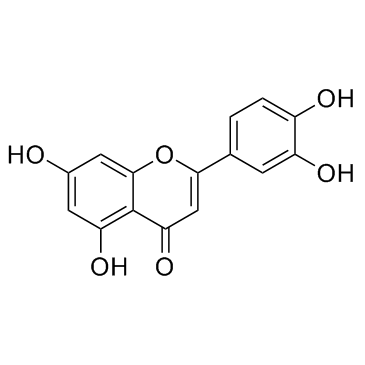 CAS#:491-70-3
CAS#:491-70-3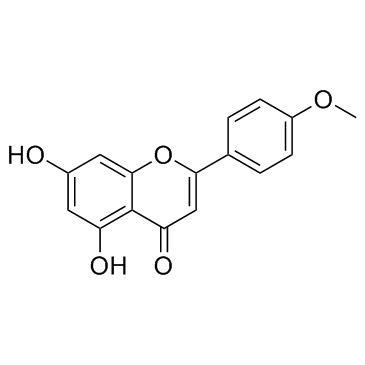 CAS#:480-44-4
CAS#:480-44-4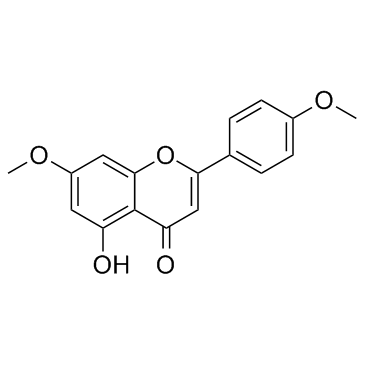 CAS#:5128-44-9
CAS#:5128-44-9 CAS#:108-73-6
CAS#:108-73-6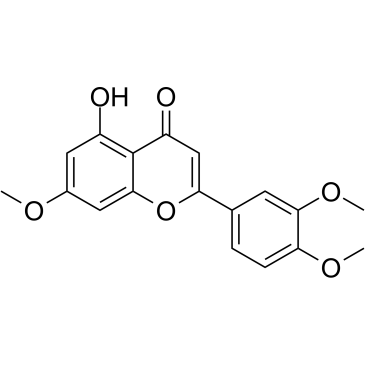 CAS#:29080-58-8
CAS#:29080-58-8![potassium 3-[5-hydroxy-2-(3-hydroxy-4-methoxy-phenyl)-4-oxo-chromen-7- yl]oxypropane-1-sulfonate structure](https://image.chemsrc.com/caspic/255/70412-86-1.png) CAS#:70412-86-1
CAS#:70412-86-1
