Fisetin
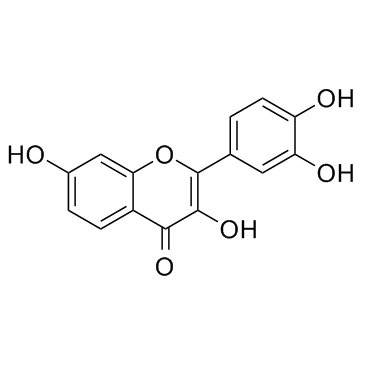
Fisetin structure
|
Common Name | Fisetin | ||
|---|---|---|---|---|
| CAS Number | 528-48-3 | Molecular Weight | 286.236 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 599.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C15H10O6 | Melting Point | 330ºC | |
| MSDS | Chinese | Flash Point | 233.0±23.6 °C | |
Use of FisetinFisetin is a natural flavonol found in many fruits and vegetables with various benefits, such as antioxidant, anticancer, neuroprotection effects. |
| Name | fisetin |
|---|---|
| Synonym | More Synonyms |
| Description | Fisetin is a natural flavonol found in many fruits and vegetables with various benefits, such as antioxidant, anticancer, neuroprotection effects. |
|---|---|
| Related Catalog | |
| Target |
Sirtuin, PPAR, TNF-alpha[1][2] |
| In Vitro | Fisetin inhibits lipid accumulation and suppresses the expression of PPARγ in 3T3-L1 cells. Fisetin suppresses early stages of preadipocyte differentiation, and induces expression of Sirt1. Fisetin facilitates Sirt1-mediated deacetylation of PPARγ and FoxO1, and enhances the association of Sirt1 with the PPARγ promoter, leading to suppression of PPARγ transcriptional activity, thereby repressing adipogenesis[1]. Fisetin binds to tubulin and stabilizes microtubules with binding characteristics far superior than paclitaxel. Fisetin treatment of human prostate cancer cells results in robust up-regulation of microtubule associated proteins (MAP)-2 and -4. Fisetin significantly inhibits PCa cell proliferation, migration, and invasion. Nudc, a protein associated with microtubule motor dynein/dynactin complex that regulates microtubule dynamics, is inhibited with Fisetin treatment[2]. |
| In Vivo | Fisetin treatment to UVB exposed mice results in decreased hyperplasia and reduces infiltration of inflammatory cells. Fisetin treatment also reduces inflammatory mediators such as COX-2, PGE2 as well as its receptors (EP1- EP4), and MPO activity. Furthermore, Fisetin reduces the level of inflammatory cytokines TNFα, IL-1β and IL-6 in UVB exposed skin. Fisetin treatment also reduces cell proliferation markers as well as DNA damage as evidenced by increased expression of p53 and p21 proteins[3]. |
| Cell Assay | 3T3-L1 cells are transfected with reporter vector, and expression plasmids using TransIT-LT1. After 24 h, media is replaced with viral supernatant. After 15-18 h of infection, media is replaced with DMI, 0.1 μM troglitazone and Fisetin (50 μM). Cells are lysed using cell culture lysis buffer two days after addition of differentiation stimulus. Luciferase activity is determined using an analytical luminescence luminometer[1]. |
| Animal Admin | Mice: The mice are divided into six groups of eight animals each. The mice in the first group are topically treated with 0.2 mL acetone and used as vehicle control. The mice in the second and third groups receive topical treatment of Fisetin 250 nmol/0.2 mL acetone/mouse and 500 nmol/0.2 mL acetone/mouse respectively on their dorsal skin and serves as agent controls. The mice in the fourth, fifth and sixth groups are exposed to UVB. The mice in the fourth group receive a topical application of 0.2 mL acetone after 15 min of UVB exposure designated as vehicle control. The mice in the fifth and sixth groups are treated with topical application of Fisetin 250 nmol/0.2 mL acetone/mouse and 500 nmol/0.2 mL acetone/mouse respectively on to their dorsal skin after 15 min of UVB exposure[3]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 599.4±50.0 °C at 760 mmHg |
| Melting Point | 330ºC |
| Molecular Formula | C15H10O6 |
| Molecular Weight | 286.236 |
| Flash Point | 233.0±23.6 °C |
| Exact Mass | 286.047729 |
| PSA | 111.13000 |
| LogP | 2.52 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.785 |
| Storage condition | −20°C |
Synonym:3,3',4',7-Tetrahydroxyflavone; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-; 5-Desoxyquercetin; C.I. 75620; C.I. Natural Brown 1; Flavone, 3,3',4',7-tetrahydroxy Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: None Listed. Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. Ingestion: May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. Inhalation: May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Chronic: No information found. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Ingestion: Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or appropriate foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 528-48-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Solid Color: ochre Odor: none reported pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: > 330 deg C Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Not available. Solubility in water: Not available. Specific Gravity/Density: Not available. Molecular Formula: C15H10O6 Molecular Weight: 286.23 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, dust generation, strong oxidants. Incompatibilities with Other Materials: Strong oxidizing agents, strong bases. Hazardous Decomposition Products: Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide. Hazardous Polymerization: Has not been reported Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 528-48-3: LK9250000 LD50/LC50: Not available. Carcinogenicity: 4H-1-benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,7-dihydroxy- - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material. Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: Not available. Risk Phrases: Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 528-48-3: No information available. Canada CAS# 528-48-3 is listed on Canada's NDSL List. CAS# 528-48-3 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 528-48-3 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~% 
Fisetin CAS#:528-48-3 |
| Literature: Hasan, Aurangzeb; Sadiq; Abbas; Mughal; Khan, Khalid M.; Ali, Muhammad Natural Product Research, 2010 , vol. 24, # 11 p. 995 - 1003 |
|
~% 
Fisetin CAS#:528-48-3 |
| Literature: Zanarotti, Antonio Heterocycles, 1982 , vol. 19, # 9 p. 1585 - 1586 |
|
~% 
Fisetin CAS#:528-48-3 |
| Literature: Bellino; Venturella Annali di Chimica (Rome, Italy), 1958 , vol. 48, p. 111,118 |
|
~% 
Fisetin CAS#:528-48-3 |
| Literature: Bellino; Venturella Annali di Chimica (Rome, Italy), 1958 , vol. 48, p. 111,118 |
| HS Code | 2914501900 |
|---|---|
| Summary | 2914501900 other ketone-phenols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
| 5-Deoxyquercetin |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,7-dihydroxy- |
| Fisetin |
| Viset |
| 3,3',4',7-tetrahydroxyflavone |
| MFCD00006829 |
| Fustet |
| Superfustel |
| Cotinin |
| Fustel |
| Fisidenolon 1521 |
| 5-Desoxyquercetin |
| 3,7-Dihydroxy-2-(3,4-dihydroxyphenyl)-4H-1-benzopyran-4-one |
| 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4-one |
| 2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one |
| Fietin |
| EINECS 208-434-4 |
| 3,7,3',4'-Tetrahydroxyflavone |
| Fisetholz |

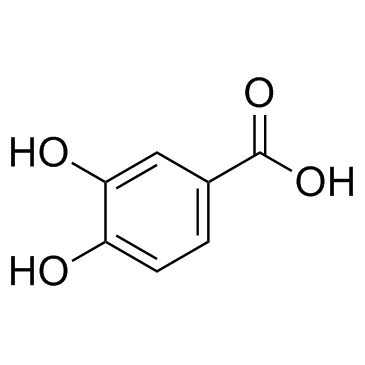 CAS#:99-50-3
CAS#:99-50-3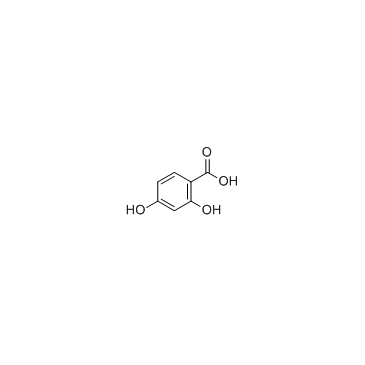 CAS#:89-86-1
CAS#:89-86-1 CAS#:108-73-6
CAS#:108-73-6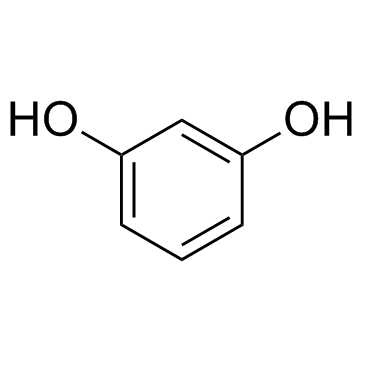 CAS#:108-46-3
CAS#:108-46-3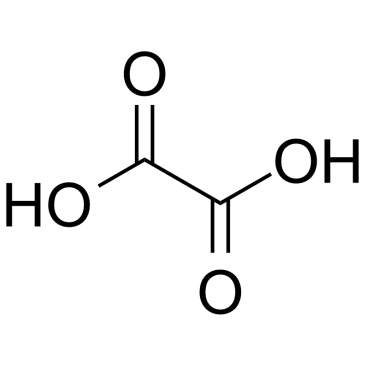 CAS#:144-62-7
CAS#:144-62-7 CAS#:88-89-1
CAS#:88-89-1
