CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
MA1225000
-
CHEMICAL NAME :
-
Glutamic acid, N-(p-(((2,4-diamino-6-pteridinyl)methyl)methylamino)b enzoyl)-, L-
-
CAS REGISTRY NUMBER :
-
59-05-2
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
143
-
MOLECULAR FORMULA :
-
C20-H22-N8-O5
-
MOLECULAR WEIGHT :
-
454.50
-
WISWESSER LINE NOTATION :
-
T66 BN DN GN JNJ CZ EZ H1N1&R DVMYVQ2VQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Non-standard exposure
-
ROUTE OF EXPOSURE :
-
Administration into the eye
-
SPECIES OBSERVED :
-
Human
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
4286 ug/kg/2.7Y-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - fibrosis, focal (pneumoconiosis) Lungs, Thorax, or Respiration - respiratory obstruction Blood - aplastic anemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2 mg/kg/17W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - other changes Blood - leukopenia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
643 ug/kg/6W-I
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
43 mg/kg/5Y
-
TOXIC EFFECTS :
-
Liver - liver function tests impaired Liver - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
2 mg/kg/12D
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - cough Lungs, Thorax, or Respiration - dyspnea Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
800 ug/kg/4D-I
-
TOXIC EFFECTS :
-
Blood - leukopenia Blood - thrombocytopenia Blood - oxidant related (GPD deficient) anemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
740 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
100 mg/kg/4H
-
TOXIC EFFECTS :
-
Blood - thrombocytopenia Blood - other changes Biochemical - Metabolism (Intermediary) - effect on inflammation or mediation of inflammation
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
4650 ug/kg/4W-I
-
TOXIC EFFECTS :
-
Liver - fatty liver degeneration Liver - liver function tests impaired
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
200 mg/kg/5Y
-
TOXIC EFFECTS :
-
Liver - hepatitis, fibrous (cirrhosis, post-necrotic scarring)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
7143 ug/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting Blood - changes in leukocyte (WBC) count Blood - changes in platelet count
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2600 ug/kg
-
TOXIC EFFECTS :
-
Brain and Coverings - changes in cerebral spinal fluid Lungs, Thorax, or Respiration - fibrosis, focal (pneumoconiosis) Lungs, Thorax, or Respiration - dyspnea
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
150 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - effect, not otherwise specified
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraspinal
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
36 mg/kg/15D
-
TOXIC EFFECTS :
-
Spinal Cord - other degenerative changes Gastrointestinal - nausea or vomiting Nutritional and Gross Metabolic - body temperature decrease
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
135 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
58 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
14 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
35 mg/kg/28W
-
TOXIC EFFECTS :
-
Vascular - BP lowering not characterized in autonomic section Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - cyanosis
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
146 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
50 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
65 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
69 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5600 ug/kg/4W-C
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Liver - hepatitis (hepatocellular necrosis), zonal Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4200 ug/kg/6W-C
-
TOXIC EFFECTS :
-
Liver - hepatitis (hepatocellular necrosis), zonal
-
TYPE OF TEST :
-
TCLo - Lowest published toxic concentration
-
ROUTE OF EXPOSURE :
-
Inhalation
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
41 mg/m3/2W-C
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
71500 ug/kg/5D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Blood - thrombocytopenia Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
7 mg/kg/12W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Blood - leukemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
125 mg/kg/6Y-I
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Liver - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
51 mg/kg/4Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Sense Organs and Special Senses (Olfaction) - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
210 mg/kg/50W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Endocrine - adrenal cortex tumors Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
139 mg/kg/55W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Lungs, Thorax, or Respiration - tumors Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
55 mg/kg/78W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Lungs, Thorax, or Respiration - tumors Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
74 mg/kg/48W-I
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Blood - lymphoma, including Hodgkin's disease Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
8260 ug/kg/44W-I
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Blood - leukemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
250 ug/kg
-
SEX/DURATION :
-
female 9 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 14-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
300 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
300 ug/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 8-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - oogenesis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
260 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - cytological changes (including somatic cell genetic material)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
female 20-24 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 11-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 17-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
600 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
600 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
9600 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
9600 ug/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
Specific locus test
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
DNA damage
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Dominant lethal test
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Mammal - species unspecified Cells - not otherwise specified
-
DOSE/DURATION :
-
1 mmol/L
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 120,139,1983 *** REVIEWS *** IARC Cancer Review:Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 26,267,1981 IARC Cancer Review:Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 26,267,1981 IARC Cancer Review:Group 3 IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,241,1987 TOXICOLOGY REVIEW NEJMAG New England Journal of Medicine. (Massachusetts Medical Soc., 10 Shattuck St., Boston, MA 02115) V.198- 1928- Volume(issue)/page/year: 291,75,1974 TOXICOLOGY REVIEW PLMJAP Pahlavi Medical Journal. (Shiraz, Iran) V.1-9, 1970-78. Volume(issue)/page/year: 6,160,1975 TOXICOLOGY REVIEW MIMDAL Minnesota Medicine. (Minnesota Medical Assoc., 2221 University Ave., SE, Suite 400, Minneapolis, MN 55414) V.1- 1918- Volume(issue)/page/year: 57,19,1974 TOXICOLOGY REVIEW JAMAAP JAMA, Journal of the American Medical Association. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1883- Volume(issue)/page/year: 172,1765,1960 TOXICOLOGY REVIEW THORA7 Thorax. (British Medical Assoc., BMA House, Travistock Square, London WC1H 9JR, UK) V.1- 1946- Volume(issue)/page/year: 27,636,1972 TOXICOLOGY REVIEW CRTXB2 CRC Critical Reviews in Toxicology. (CRC Press, Inc., 2000 Corporate Blvd., NW, Boca Raton, FL 33431) V.1- 1971- Volume(issue)/page/year: 2,159,1973 TOXICOLOGY REVIEW MJAUAJ Medical Journal of Australia. (Australasian Medical Pub. Co. Ltd., 71-79 Arundel St., Glebe, N.S.W., Australia) V.1- 1914- Volume(issue)/page/year: 2,1076,1971 TOXICOLOGY REVIEW MEDIAV Medicine. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1922- Volume(issue)/page/year: 55,371,1976 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X3716 No. of Facilities: 529 (estimated) No. of Industries: 2 No. of Occupations: 24 No. of Employees: 17193 (estimated) No. of Female Employees: 9496 (estimated)
|
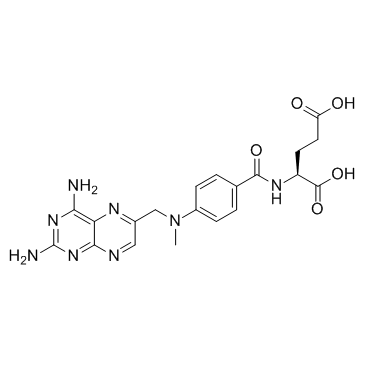




 CAS#:56-86-0
CAS#:56-86-0![4-{[N-[(2,4-diamino-6-pteridinyl)methyl]-N-methyl]amino}benzoyl azide Structure](https://image.chemsrc.com/caspic/445/56892-87-6.png) CAS#:56892-87-6
CAS#:56892-87-6![3-[3-((allyloxy)carbonyl)-5-oxo-1,3-oxazolan-4-yl]propanoic acid Structure](https://image.chemsrc.com/caspic/069/138965-46-5.png) CAS#:138965-46-5
CAS#:138965-46-5 CAS#:91871-28-2
CAS#:91871-28-2![Diethyl N-[4-(methylamino)benzoyl]-L-glutamate Structure](https://image.chemsrc.com/caspic/000/2378-95-2.png) CAS#:2378-95-2
CAS#:2378-95-2 CAS#:7732-18-5
CAS#:7732-18-5 CAS#:52853-40-4
CAS#:52853-40-4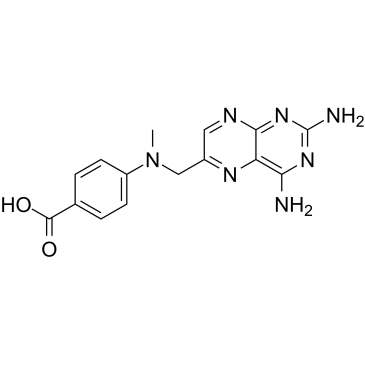 CAS#:19741-14-1
CAS#:19741-14-1![ditert-butyl 2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]pentanedioate Structure](https://image.chemsrc.com/caspic/063/86669-33-2.png) CAS#:86669-33-2
CAS#:86669-33-2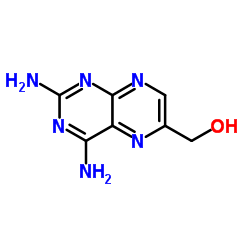 CAS#:945-24-4
CAS#:945-24-4![N-[4-(Methylamino)benzoyl]-L-glutamic Acid structure](https://image.chemsrc.com/caspic/434/52980-68-4.png) CAS#:52980-68-4
CAS#:52980-68-4![2-[[4-[(2-Amino-4-Oxo-1H-Pteridin-6-Yl)Methyl-Methylamino]Benzoyl]Amino]Pentanedioic Acid structure](https://image.chemsrc.com/caspic/124/2410-93-7.png) CAS#:2410-93-7
CAS#:2410-93-7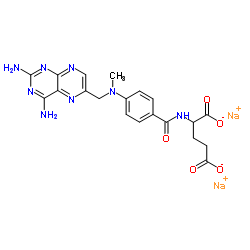 CAS#:7413-34-5
CAS#:7413-34-5![(2S)-2-[[3-bromo-4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]pentanedioic acid structure](https://image.chemsrc.com/caspic/237/13082-83-2.png) CAS#:13082-83-2
CAS#:13082-83-2![bis[(6-chlorobenzo[1,3]dioxol-5-yl)methyl] 2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]pentanedioate structure](https://image.chemsrc.com/caspic/145/86688-51-9.png) CAS#:86688-51-9
CAS#:86688-51-9![1-[(4-{[(2,4-Diaminopteridin-6-yl)methyl]methylamino}-5-bromo-3-chlorophenyl)carbonylamino]propane-1,3-dicarboxylic acid structure](https://image.chemsrc.com/caspic/256/6914-12-1.png) CAS#:6914-12-1
CAS#:6914-12-1
