CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
EK7798000
-
CHEMICAL NAME :
-
Butanoic acid, 2,2-dimethyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro- 4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester, (1S-(1-alpha,3-alpha,7- beta,8-beta(2S*,4S*),8a-beta))-
-
CAS REGISTRY NUMBER :
-
79902-63-9
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C25-H38-O5
-
MOLECULAR WEIGHT :
-
418.63
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2800 ug/kg/7D-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Liver - jaundice (or hyperbilirubinemia) hepatocellular Liver - liver function tests impaired
-
REFERENCE :
-
MJAUAJ Medical Journal of Australia. (Australasian Medical Pub. Co. Ltd., 71-79 Arundel St., Glebe, N.S.W., Australia) V.1- 1914- Volume(issue)/page/year: 155,61,1991
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
108 mg/kg/77W-I
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Lungs, Thorax, or Respiration - acute pulmonary edema Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
ANZJB8 Australian and New Zealand Journal of Medicine. (Modern Medicine of Australia Pty., Ltd., 100 Pacific Highway, North Sydney, 2060, Australia) V.1- 1971- Volume(issue)/page/year: 25,745,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4438 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Gastrointestinal - other changes
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,95,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
705 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Behavioral - muscle contraction or spasticity
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,95,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
672 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - muscle contraction or spasticity
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,95,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - somnolence (general depressed activity) Gastrointestinal - other changes
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,95,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
798 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Behavioral - muscle contraction or spasticity
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,95,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1009 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - muscle contraction or spasticity
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,95,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,95,1990 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
9275 mg/kg/53W-I
-
TOXIC EFFECTS :
-
Brain and Coverings - changes in brain weight Liver - changes in liver weight Endocrine - changes in thyroid weight
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,103,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
17640 mg/kg/14W-I
-
TOXIC EFFECTS :
-
Blood - changes in bone marrow (not otherwise specified) Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases Related to Chronic Data - death
-
REFERENCE :
-
YKYUA6 Yakkyoku. Pharmacy. (Nanzando, 4-1-11, Yushima, Bunkyo-ku, Tokyo, Japan) V.1- 1950- Volume(issue)/page/year: 43,259,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
120 mg/kg/12D-I
-
TOXIC EFFECTS :
-
Liver - hepatitis (hepatocellular necrosis), zonal Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
REFERENCE :
-
PHTOEH Pharmacology and Toxicology (Copenhagen). (Munksgaard International Pub., POB 2148, DK-1016 Copenhagen K, Denmark) V.60- 1987- Volume(issue)/page/year: 77,391,1995 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 6-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,143,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
480 mg/kg
-
SEX/DURATION :
-
female 6-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,143,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
350 mg/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 39,169,1990
|
 CAS#:139893-43-9
CAS#:139893-43-9 CAS#:145576-25-6
CAS#:145576-25-6 CAS#:272456-97-0
CAS#:272456-97-0 CAS#:18820-82-1
CAS#:18820-82-1 CAS#:75330-75-5
CAS#:75330-75-5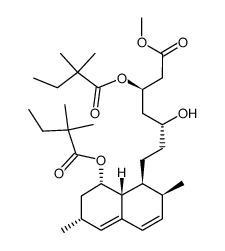 CAS#:882025-44-7
CAS#:882025-44-7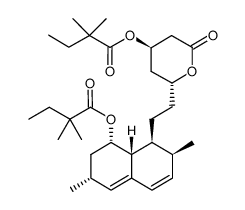 CAS#:851402-85-2
CAS#:851402-85-2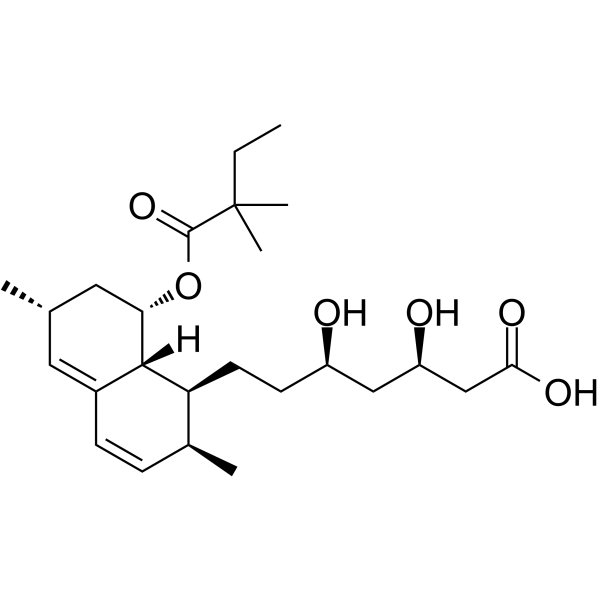 CAS#:121009-77-6
CAS#:121009-77-6![6(R)-[2-[8(S)-((2,2-Dimethylbutyryl)oxy)-2(S),6(R)-dimethyl-1,2,6,7,8,8a(R)-hexahydronaphth-1(S)-yl]ethyl]-4(R)-((tert-butyldimethylsilyl)oxy)-3,4,5,6-tetrahydro-2H-pyran-2-one Structure](https://image.chemsrc.com/caspic/186/79902-59-3.png) CAS#:79902-59-3
CAS#:79902-59-3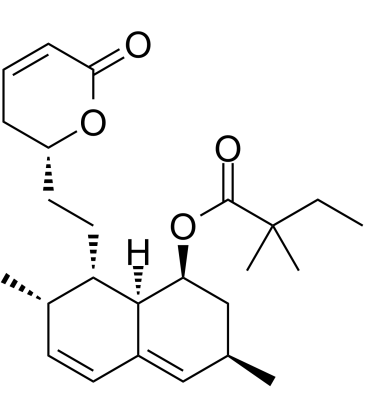 CAS#:210980-68-0
CAS#:210980-68-0 CAS#:67-64-1
CAS#:67-64-1 CAS#:75-65-0
CAS#:75-65-0 CAS#:101314-97-0
CAS#:101314-97-0![[(1S,3R,4S,4aS,7S,8S,8aS)-8-[2-[(2R,4R)-4-[tert-butyl(dimethyl)silyl]oxy-6-oxooxan-2-yl]ethyl]-4-chloro-4a-hydroxy-3,7-dimethyl-2,3,4,7,8,8a-hexahydro-1H-naphthalen-1-yl] 2,2-dimethylbutanoate structure](https://image.chemsrc.com/caspic/192/123852-10-8.png) CAS#:123852-10-8
CAS#:123852-10-8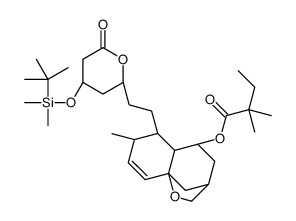 CAS#:125175-64-6
CAS#:125175-64-6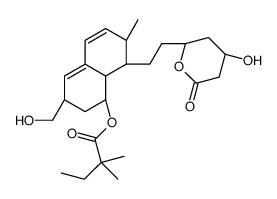 CAS#:114883-29-3
CAS#:114883-29-3
