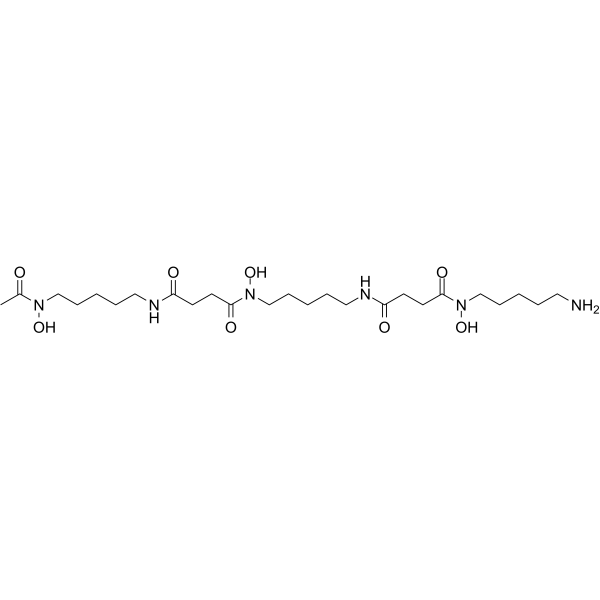
70-51-9
| 中文名 | 去铁胺 |
|---|---|
| 英文名 | desferrioxamine B |
| 中文别名 | 去铁敏 |
| 英文别名 |
desferrioxamine mesylate
Deferoxamin EINECS 201-064-4 Desferal Deferrioxamine B Deferrioxamine Desferin DESFERRIOXAMINE deferoxamine MFCD00004679 Deferoxamine B Desferrioxamine B N-[5-[[4-[5-[acetyl(hydroxy)amino]pentylamino]-4-oxobutanoyl]-hydroxyamino]pentyl]-N'-(5-aminopentyl)-N'-hydroxybutanediamide Deferoxaminum |
| 描述 | 去铁胺(去铁胺B)是一种铁螯合剂(与Fe(III)和许多其他金属阳离子结合),广泛用于减少组织中的铁积累和沉积。去铁胺通过良好的抗氧化活性上调HIF-1α水平。去铁胺还具有抗增殖活性,可诱导癌细胞凋亡和自噬。去铁胺可用于糖尿病、神经退行性疾病以及抗癌和抗新冠肺炎[1][2][3][4][5]的研究。 |
|---|---|
| 相关类别 | |
| 体外研究 | 去铁胺(1mM;16小时或4周)改善缺氧和高血糖条件下的HIF-1α功能,并减少MEFs细胞中的ROS[1]。去铁胺(100µM;24小时)增加InsR表达和活性,并诱导p-Akt/总Akt/PKB水平的增加[2]。去铁胺(5、10、25、50、100µM;7或9天)抑制肿瘤相关MSC和骨髓MSC的增殖[3]。去铁胺(5、10、25、50、100µM;7天)诱导骨髓间充质干细胞凋亡[3]。去铁胺(10µM;3天)影响MSC上粘附蛋白的表达[3]。去铁胺(100µM;24小时)诱导SH-SY5Y细胞中HIF-1α水平介导的自噬[4]。Western印迹分析[1]细胞系:MEFs细胞浓度:1mM,孵育时间:16h(缺氧条件);4周(高血糖状态)结果:显著降低低氧高糖条件下高血糖相关的ROS水平升高。在高糖和正常葡萄糖条件下,MEF中的常氧HIF反式激活显著增加。Western Blot分析[2]细胞系:HepG2细胞浓度:100µM孵育时间:24小时结果:细胞内InsR mRNA水平增加两倍。在1.1nM的半最大浓度下增加了两倍的InsR结合活性。细胞增殖测定[3]细胞系:TAMSCs和BMMSCs(均从雄性C57BL/6J小鼠(8周龄;EG-7诱导的肿瘤模型)中分离)浓度:5、10、25、50、100µM,孵育时间:7天(TAMSCs);9天(骨髓间充质干细胞)。结果:抑制TAMSC和BMMSC的生长,当暴露于50和100µM剂量时,大多数细胞在第7天或第9天死亡。凋亡分析[3]细胞系:TAMSCs,BMMSCs浓度:5、10、25、50、100µM孵育时间:7天结果:对TAMSCs和BMMSCs细胞显示促凋亡作用。Western Blot分析[3]细胞系:TAMSCs,BMMSCs浓度:10µM孵育时间:3天结果:TAMSC和BMMSCs中VCAM-1表达显著降低。细胞自噬测定[4]细胞系:SH-SY5Y细胞浓度:100µM孵育时间:24小时结果:增加了自噬指标LC3-II/I的比率,当自噬相关基因Beclin 1被Beclin 1siRNA转染抑制时,这种效应被阻断。导致HIF-1a的时间和剂量依赖性增加,同时诱导自噬。 |
| 体内研究 | 去铁胺(560.68 mg/per;滴注;每日一次,持续21天)可促进老年或糖尿病小鼠的伤口愈合并增加新生血管[1]。去铁胺(200mg/kg;腹腔注射;每日2周)导致HIF-1α稳定,并增加体内葡萄糖摄取、肝脏InsR表达和信号[2]。动物模型:老年(21月龄)和糖尿病(12周龄)C57BL/6J小鼠(切除伤口模型)[1]。剂量:560.68毫克/每(10微升1mM)给药:滴注;每天一次,持续21天。结果:在老年和糖尿病小鼠模型中均显示出明显的加速愈合和增加的新生血管。动物模型:雄性Sprague-Dawley大鼠(180-200g)[2]。剂量:200mg/kg给药:腹腔注射;每日2周。结果:肝脏中显著升高的HIF-1α蛋白水平、InsR蛋白水平以及Akt/PKB和激活的Akt/PKB在肝脏中显著增高。 |
| 参考文献 |
| 密度 | 1.212g/cm3 |
|---|---|
| 沸点 | 627.9°C (rough estimate) |
| 熔点 | 139°C |
| 分子式 | C25H48N6O8 |
| 分子量 | 560.68400 |
| 精确质量 | 560.35300 |
| PSA | 205.84000 |
| LogP | 2.40420 |
| 折射率 | 1.537 |
| 储存条件 | 保存方法;远离阳光在寒冷的“ ( < -20 ℃ = -4)通风干燥的地方。保持容器密闭。 |
| 稳定性 | 不能与氧化剂共存 |
| 分子结构 | 1、 摩尔折射率:144.51 2、 摩尔体积(m3/mol):462.3 3、 等张比容(90.2K):1264.2 4、 表面张力(dyne/cm):55.8 5、 极化率(10 -24cm 3):57.28 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):-2.1 2.氢键供体数量:6 3.氢键受体数量:9 4.可旋转化学键数量:23 5.互变异构体数量:4 6.拓扑分子极性表面积206 7.重原子数量:39 8.表面电荷:0 9.复杂度:739 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:白色-近白色,晶体-粉 2. 密度(g/mL,25/4℃):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):未确定 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,5.2kPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:。未确定 |
|
生态学数据: 该物质对水体有一定的危害,防止泄漏进入下水道,水道或低的地区。 CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 海关编码 | 2924199090 |
|---|
| 海关编码 | 2924199090 |
|---|---|
| 中文概述 | 2924199090. 其他无环酰胺(包括无环氨基甲酸酯)(包括其衍生物及盐). 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途, 包装 |
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |