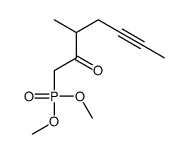
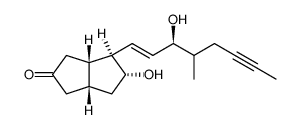
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
SA3695000
-
CHEMICAL NAME :
-
Pentanoic acid, 5-(hexahydro-5-hydroxy-4-(3-hydroxy-4-methyl-1-octen- 6-ynyl)-2(1H)- pentalenylidene)-
-
CAS REGISTRY NUMBER :
-
78919-13-8
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
10
-
MOLECULAR FORMULA :
-
C22-H32-O4
-
MOLECULAR WEIGHT :
-
360.54
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRFUD4 Drugs of the Future. (J.R. Prous, S.A., Apartado de Correos 540, 08080 Barcelona, Spain) V.1- 1975/76- Volume(issue)/page/year: 6,676,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
119 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - muscle contraction or spasticity Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
NCDREP New Cardiovascular Drugs. (Raven Press, 1185 Ave. of the Americas, New York, NY 10036) 1985- Volume(issue)/page/year: 4,209,1986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRFUD4 Drugs of the Future. (J.R. Prous, S.A., Apartado de Correos 540, 08080 Barcelona, Spain) V.1- 1975/76- Volume(issue)/page/year: 6,676,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
201 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - muscle contraction or spasticity Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
NCDREP New Cardiovascular Drugs. (Raven Press, 1185 Ave. of the Americas, New York, NY 10036) 1985- Volume(issue)/page/year: 4,209,1986 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
21780 ug/kg/11D-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Behavioral - somnolence (general depressed activity) Blood - other changes
-
REFERENCE :
-
NCDREP New Cardiovascular Drugs. (Raven Press, 1185 Ave. of the Americas, New York, NY 10036) 1985- Volume(issue)/page/year: 4,209,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
120 mg/kg/28D-C
-
TOXIC EFFECTS :
-
Related to Chronic Data - death
-
REFERENCE :
-
NCDREP New Cardiovascular Drugs. (Raven Press, 1185 Ave. of the Americas, New York, NY 10036) 1985- Volume(issue)/page/year: 4,209,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
4760 ug/kg/28D-C
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Vascular - BP lowering not characterized in autonomic section Related to Chronic Data - death
-
REFERENCE :
-
NCDREP New Cardiovascular Drugs. (Raven Press, 1185 Ave. of the Americas, New York, NY 10036) 1985- Volume(issue)/page/year: 4,209,1986 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
10 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
TOLED5 Toxicology Letters. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1977- Volume(issue)/page/year: 78,223,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
26100 ug/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception lactating female 22 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
REFERENCE :
-
TOLED5 Toxicology Letters. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1977- Volume(issue)/page/year: 78,223,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
26100 ug/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception lactating female 22 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
TOLED5 Toxicology Letters. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1977- Volume(issue)/page/year: 78,223,1995
|


![Methyl (1S,5S,6R,7R)-7-Hydroxy-6-[3(R)-hydroxy-4-methyl-E-1-octen-6-ynyl]bicyclo[3.3.0]octane-E-Δ3,δ-pentanoate结构式](https://image.chemsrc.com/caspic/139/101314-49-2.png)