1355149-45-9
| Name | GS 441524 triphosphate |
|---|---|
| Synonyms |
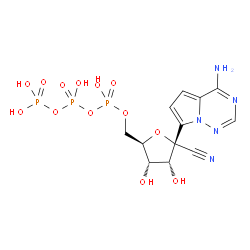
((2R,3S,4R,5R)-5-(4-Aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methyl tetrahydrogen triphosphate
GS-441524 |
| Description | GS-443902 (Remdesivir metabolite) is a potent viral RNA-dependent RNA-polymerases (RdRp) inhibitor with IC50s of 5.6 µM, 1.1 µM, 5 µM for TP RdRp, RSV RdRp and HCV RdRp, respectively. GS-443902 is the active triphosphate metabolite of Remdesivir[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 5.6 µM (TP RdRp), 1.1 µM (RSV RdRp) and 5 µM (HCV RdRp)[1][2] |
| In Vitro | GS-443902 (compound 8a), a triphosphates (TP) derivative, is a potent inhibitory activity against the enzyme TP RdRp (IC50=5.6 µM)[1]. In a continuous 72 h incubation of 1 µM GS-5734, the GS-443902 (compound 4tp) level is measured at 2, 24, 48 and 72 h, and reaches a Cmax of 300, 110, and 90 pmol/million cells in macrophages, HMVEC, and HeLa cells lines respectively[2]. GS-443902 (NTP; 0.01, 0.1, 1, 10, 100 μM) inhibits RSV RdRp-catalysed RNA synthesis by incorporating into the nascent viral RNA transcript and causing its premature termination. GS-5734 selectively inhibits EBOV replication by targeting its RdRp and inhibiting viral RNA synthesis following efficient intracellular conversion to GS-443902[3]. |
| In Vivo | GS-5734 (10 mg kg; i.v.) rapidly distributes into peripheral blood mononuclear cells (PBMCs), and efficient conversion to GS-443902 (NTP) is apparent within 2 h of dose administration in rhesus monkeys. In PBMCs, GS-443902 represents the predominant metabolite and is persistent with a t1/2 of 14 h and levels required for >50% virus inhibition for 24 hours[3]. |
| References |
| Density | 2.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C12H16N5O13P3 |
| Molecular Weight | 531.202 |
| Exact Mass | 530.995728 |
| LogP | -5.92 |
| Index of Refraction | 1.841 |
| Storage condition | -20°C |