CM-272
Modify Date: 2024-01-11 11:15:23
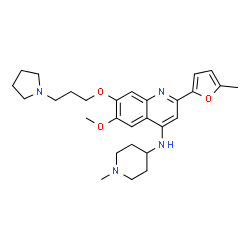
CM-272 structure
|
Common Name | CM-272 | ||
|---|---|---|---|---|
| CAS Number | 1846570-31-7 | Molecular Weight | 478.626 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 631.9±55.0 °C at 760 mmHg | |
| Molecular Formula | C28H38N4O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 336.0±31.5 °C | |
Use of CM-272CM-272 (CM272) is a novel potent, selective and reversible dual inhibitor of G9a/DNMTs with IC50 of 8 nM and 382 nM for G9a and DNMT1, respectively; also inhibits DNMT3A/3B/GLP with IC50 of 85/1,200/2 nM, respectively; inhibits cell proliferation and promotes apoptosis in different haematological neoplasias (AML, ALL and DLBCL); significantly prolongs survival of AML, ALL and DLBCL xenogeneic models. |
| Name | 6-Methoxy-2-(5-methyl-2-furyl)-N-(1-methyl-4-piperidinyl)-7-[3-(1-pyrrolidinyl)propoxy]-4-quinolinamine |
|---|---|
| Synonym | More Synonyms |
| Description | CM-272 (CM272) is a novel potent, selective and reversible dual inhibitor of G9a/DNMTs with IC50 of 8 nM and 382 nM for G9a and DNMT1, respectively; also inhibits DNMT3A/3B/GLP with IC50 of 85/1,200/2 nM, respectively; inhibits cell proliferation and promotes apoptosis in different haematological neoplasias (AML, ALL and DLBCL); significantly prolongs survival of AML, ALL and DLBCL xenogeneic models. |
|---|---|
| Related Catalog | |
| Target |
G9a:8 nM (IC50) GLP:2 nM (IC50) DNMT1:382 nM (IC50) DNMT3A:85 nM (IC50) DNMT3B:1200 nM (IC50) |
| In Vitro | CM-272 (100-1000 nM; 12-72 hours; CEMO-1, MV4-11 and OCI-Ly10 cell lines) treatment inhibits cell proliferation in a dose- and time-dependent manner[1]. CM-272 (100-1000 nM; 24 hours; CEMO-1, MV4-11 and OCI-Ly10 cell lines) treatment blocks cell cycle progression[1]. CM-272 (100-1000 nM; 12-72 hours; CEMO-1, MV4-11 and OCI-Ly10 cell lines) treatment induces apoptosis in ALL, AML and DLBCL cell lines in a dose- and time-dependent manner[1]. CM-272 after 48 h of treatment CEMO-1 acute lymphoblastic leukaemia (ALL) cell line, MV4-11 acute myeloid leukaemia (AML) cell line and OCI-Ly10 diffuse large B-cell lymphoma (DLBCL) cell line, the GI50 values of 218 nM, 269 nM and 455 nM, respectively, and is associated with a decrease in global levels of H3K9me2 and 5mC[1]. The therapeutic activity of CM-272 relies on the early activation of the type I IFN response in tumour cells, potentially leading to the induction of cell autonomous immunogenic death in tumour cells[1]. Cell Proliferation Assay[1] Cell Line: CEMO-1, MV4-11 and OCI-Ly10 cell lines Concentration: 125 nM, 250 nM, 500 nM (CEMO-1 cells); 135 nM, 270 nM, 540 nM (MV4-11 cells); 100 nM, 400 nM, 1000 nM (OCI-Ly10 cells) Incubation Time: 12 hours, 24 hours, 48 hours and 72 hours Result: Inhibited cell proliferation in a dose- and time-dependent manner. Cell Cycle Analysis[1] Cell Line: CEMO-1, MV4-11 and OCI-Ly10 cell lines Concentration: 125 nM, 250 nM, 500 nM (CEMO-1 cells); 135 nM, 270 nM, 540 nM (MV4-11 cells); 100 nM, 400 nM, 1000 nM (OCI-Ly10 cells) Incubation Time: 24 hours Result: Blocked cell cycle progression. Apoptosis Analysis[1] Cell Line: CEMO-1, MV4-11 and OCI-Ly10 cell lines Concentration: 125 nM, 250 nM, 500 nM (CEMO-1 cells); 135 nM, 270 nM, 540 nM (MV4-11 cells); 100 nM, 400 nM, 1000 nM (OCI-Ly10 cells) Incubation Time: 12 hours, 24 hours, 48 hours and 72 hours Result: Induced apoptosis in ALL, AML and DLBCL cell lines in a dose- and time-dependent manner. |
| In Vivo | CM-272 (2.5 mg/kg; intravenous injection; daily; for 28 days; female Rag2−/−γc−/− mice) treatment significantly prolongs survival of CEMO-1 cells xenogeneic models[1]. Animal Model: Female BALB/Ca-Rag2−/−γc−/− mice (6–8-week-old) with CEMO-1 cells[1] Dosage: 2.5 mg/kg Administration: Intravenous injection; daily; for 28 days Result: Induced a statistically significant increase in overall survival (OS) in mice. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 631.9±55.0 °C at 760 mmHg |
| Molecular Formula | C28H38N4O3 |
| Molecular Weight | 478.626 |
| Flash Point | 336.0±31.5 °C |
| Exact Mass | 478.294403 |
| LogP | 5.41 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.601 |
| 6-Methoxy-2-(5-methyl-2-furyl)-N-(1-methyl-4-piperidinyl)-7-[3-(1-pyrrolidinyl)propoxy]-4-quinolinamine |
| 4-Quinolinamine, 6-methoxy-2-(5-methyl-2-furanyl)-N-(1-methyl-4-piperidinyl)-7-[3-(1-pyrrolidinyl)propoxy]- |