Bergapten

Bergapten structure
|
Common Name | Bergapten | ||
|---|---|---|---|---|
| CAS Number | 484-20-8 | Molecular Weight | 216.189 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 412.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C12H8O4 | Melting Point | 190-193 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 203.2±28.7 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of BergaptenBergapten is a natural anti-inflammatory and anti-tumor agent isolated from bergamot essential oil, other citrus essential oils and grapefruit juice. Bergapten is inhibitory towards mouse and human CYP isoforms. |
| Name | 5-methoxypsoralen |
|---|---|
| Synonym | More Synonyms |
| Description | Bergapten is a natural anti-inflammatory and anti-tumor agent isolated from bergamot essential oil, other citrus essential oils and grapefruit juice. Bergapten is inhibitory towards mouse and human CYP isoforms. |
|---|---|
| Related Catalog | |
| Target |
CYP[1] |
| In Vitro | There is decreased N-acetyltransferase (NAT) activity in SC-M1 cells at concentrations of Bergapten (5-Methoxypsoralen, 5-MOP) from 0.05 mM to 25 mM, but no obvious dose-dependent effect is found between these doses (r=0.5687). In COLO 205 cells, there is decreased NAT activity at low doses of Bergapten (0.05 mM and 0.5 mM) and increased NAT activity at a high dose (50 mM). Bergapten induces a dosedependent effect in our experimental concentrations on COLO 205 cells (r=0.8912); a promotion effect at a higher dose (50 mM) and an inhibition effect at lower doses (0.05-0.5 mM), while the concentrations 5-25 mM has no significant difference compared with the control regimen[1]. Bergapten (5-Methoxypsoralen) exerts inhibitory effects on diabetes-related osteoporosis via the regulation of the PI3K/AKT, JNK/MAPK and NF-κB signaling pathways in osteoprotegerin knockout mice. Bergapten has also been shown to significantly inhibit the production of pro-inflammatory cytokines. Bergapten exhibits the ability to significantly inhibit RANKL-RANK signaling transduction, and to suppress the activation of the PI3K/AKT, JNK/MAPK and NF-κB signaling pathways, thus protecting trabecular structure and decreasing osteoclastogenic differentiation[2]. |
| In Vivo | The metabolic activity of NAT of the rat colon is higher than that of the stomach, and Bergapten (5-Methoxypsoralen, 5-MOP) causes a decrease of AF concentration in the stomach at the 24-h time-period. The concentrations of AAF in the stomach and colon are low. Although DMSO (solvent) influenced the metabolism of AAF, compared with the control regimen, Bergapten still causes an increase in the further metabolism of AAF, and a decrease in the concentration of AAF in the stomach at 24 h, and in the colon during the 24- to 72-h time-period[1]. |
| Cell Assay | The human colon adenocarcinoma cell line (COLO 205, from a 70-year-old male Caucasian) sre placed into 75-cm2 tissue culture flasks and grown in RPMI1640 medium, supplemented with 10% fetal bovine serum, containing penicillin and streptomycin (100 μg/mL) and 1 mM glutamine, at 37°C in a humidified atmosphere of 5% CO2 and 95% O2. The human stomach adenocarcinoma cell line are placed into 75-cm2 tissue culture flasks and grown in RPMI 1640 medium, supplemented with 10% fetal bovine serum, containing penicillin and streptomycin (100 μg/mL) and 1 mM glutamine, at 37°C in a humidified atmosphere of 5% CO2 and 95% O2. SC-M1 and COLO 205 cells are treated with different concentrations of Bergapten (0.05, 0.5, 5, 10, 25 and 50 mM) and incubated for 72 h for the dose-effect study of Bergapten on NAT activity. To determine the time-course effect of 0.5 mM Bergapten on NAT activity, the cells are incubated at 37AC and harvested at 12, 24, 48 and 72 h, respectively. Bergapten is dissolved in DMSO and the final concentration of vehicle is <0.1%. Only DMSO (solvent) is added for the control regimen[1]. |
| Animal Admin | Rats[1] Seventy-two male Sprague-Dawley (SD) rats, weighing approximately 200 g are used. A total of 72 rats are subjected to 3 different regimens, each regimen divided into 4 groups with 6 rats in each group. Gastric intubation is used for delivery of the test compounds into each animal. The first regimen received 1 mL Bergapten (dissolved in DMSO) at a dose of 0.5 mmol per Kg of body weight. Regimen 2, the control regimen, received only 1 mL solvent (DMSO), without any Bergapten. Regimen 3, the contrast regimen, received nothing at that time. Twenty-four h later, all of the rats from the 3 regimens received 1mL AF (dissolved in DMSO) at a dose of 0.3 mmol per Kg of body weight. The groups are divided by different collecting time: 12, 24, 48 and 72 h after AF administration, and then the animals are transferred to individual metabolism cages. The stomachs and the colons of the rats from each regimen are collected and are immediately extracted with ethyl acetate/methanol (95:5). The solvent is evaporated and the residue redissolved in methanol and assayed for AF and AAF by HPLC. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 412.4±45.0 °C at 760 mmHg |
| Melting Point | 190-193 °C(lit.) |
| Molecular Formula | C12H8O4 |
| Molecular Weight | 216.189 |
| Flash Point | 203.2±28.7 °C |
| Exact Mass | 216.042252 |
| PSA | 52.58000 |
| LogP | 2.00 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.635 |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. May be light sensitive. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H334-H340-H350 |
| Precautionary Statements | P201-P261-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R43 |
| Safety Phrases | S36/37 |
| RIDADR | UN 1759 |
| WGK Germany | 2 |
| RTECS | LV1300000 |
| HS Code | 2932999099 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Non-animal photosafety screening for complex cosmetic ingredients with photochemical and photobiochemical assessment tools.
Regul Toxicol Pharmacol 72 , 578-85, (2015) Previously, a non-animal screening approach was proposed for evaluating photosafety of cosmetic ingredients by means of in vitro photochemical and photobiochemical assays; however, complex cosmetic in... |
|
|
Bergapten drives autophagy through the up-regulation of PTEN expression in breast cancer cells.
Mol. Cancer 14 , 130, (2015) Bergapten (5-methoxypsoralen), a natural psoralen derivative present in many fruits and vegetables, has shown antitumoral effects in a variety of cell types. In this study, it has been addressed how B... |
|
|
[Chemical constituents contained in seeds of Notopterygium franchetii].
Zhongguo Zhong Yao Za Zhi 37(7) , 941-5, (2012) To study the chemical constituents from the seeds of Notopterygium franchetii.Ethanol extracts of seeds N. franchetii were separated and purified by such methods as normal and reversed phase column ch... |
| Geralen |
| Heraclin |
| Bergapten |
| EINECS 207-604-5 |
| 4-MOP |
| Bergaptan |
| MFCD00010272 |
| 4-Methoxyfuro[3,2-g]benzopyrane-7-one,5-Methoxypsoralen,Bergapten |
| 7H-Furo[3,2-g][1]benzopyran-7-one, 4-methoxy- |
| MAJUDIN |
| 4-Methoxy-7H-furo[3,2-g][1]benzopyran-7-one |
| 5-Methoxypsoralen |
| 4-Methoxyfuro[3,2-g]Benzopyran-7-One |
| 5-MOP |
| 5-19-06-00004 (Beilstein Handbook Reference) |
| 4-Methoxy-furo[3,2-g]chromen-7-one |
| Psoraderm |
| 4-Methoxy-7H-furo[3,2-g]chromen-7-one |
 CAS#:108-73-6
CAS#:108-73-6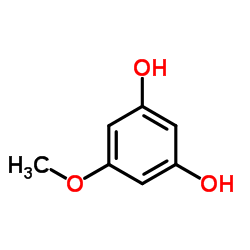 CAS#:2174-64-3
CAS#:2174-64-3 CAS#:3067-10-5
CAS#:3067-10-5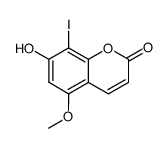 CAS#:74794-29-9
CAS#:74794-29-9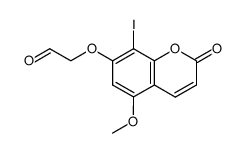 CAS#:866081-74-5
CAS#:866081-74-5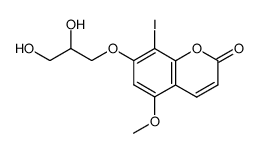 CAS#:866081-73-4
CAS#:866081-73-4 CAS#:486-60-2
CAS#:486-60-2 CAS#:77-78-1
CAS#:77-78-1 CAS#:482-45-1
CAS#:482-45-1 CAS#:870653-45-5
CAS#:870653-45-5 CAS#:737-52-0
CAS#:737-52-0 CAS#:7380-40-7
CAS#:7380-40-7 CAS#:74-88-4
CAS#:74-88-4 CAS#:3067-09-2
CAS#:3067-09-2 CAS#:145414-76-2
CAS#:145414-76-2 CAS#:2643-85-8
CAS#:2643-85-8
