NH2-C6-NH-Boc

NH2-C6-NH-Boc structure
|
Common Name | NH2-C6-NH-Boc | ||
|---|---|---|---|---|
| CAS Number | 51857-17-1 | Molecular Weight | 216.320 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 325.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C11H24N2O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 150.5±23.2 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
Use of NH2-C6-NH-BocNH2-C6-NH-Boc is a PROTAC linker which refers to the alkyl/ether composition. NH2-C6-NH-Boc can be used in the synthesis the Mcl-1 inhibitor based on PROTAC[1]. |
| Name | N-tert-Butoxycarbonyl-1,6-hexanediamine |
|---|---|
| Synonym | More Synonyms |
| Description | NH2-C6-NH-Boc is a PROTAC linker which refers to the alkyl/ether composition. NH2-C6-NH-Boc can be used in the synthesis the Mcl-1 inhibitor based on PROTAC[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 325.3±25.0 °C at 760 mmHg |
| Molecular Formula | C11H24N2O2 |
| Molecular Weight | 216.320 |
| Flash Point | 150.5±23.2 °C |
| Exact Mass | 216.183777 |
| PSA | 64.35000 |
| LogP | 1.82 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.460 |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C:Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S26-S36/37/39-S45 |
| RIDADR | UN 2735 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2924199090 |
| Precursor 10 | |
|---|---|
| DownStream 7 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Carboxymethylcellulose-tetrahydrocurcumin conjugates for colon-specific delivery of a novel anti-cancer agent, 4-amino tetrahydrocurcumin.
Eur. J. Pharm. Biopharm. 88(2) , 351-60, (2014) Several curcumin derivatives are now becoming increasingly of interest because of their bioactive attributes, especially their action as antioxidants and anti-carcinogenic activities. Tetrahydrocurcum... |
|
|
Structure-activity relationships of novel vasopressin antagonists containing C-terminal diaminoalkanes and (aminoalkyl)guanidines.
J. Med. Chem. 32 , 391, (1989) We report the synthesis and biological activity of a series of analogues of the vasopressin antagonists [Pmp1,D-Tyr(Et)2,Val4]arginine-vasopressin (1) and [Pmp1,D-Tyr(Et)2,Val4,desGly9]arginine-vasopr... |
|
|
O. Buchardt et al.
J. Org. Chem. 49 , 4123, (1984)
|
| N-(tert-Butoxycarbonyl)-1,6-diaminohexane |
| N-Boc-1,6-hexanediamine |
| tert-Butyl (6-aminohexyl)carbamate |
| N-Boc-1,6-Diaminohexane |
| N-(6-Aminohexyl)carbamic Acid tert-Butyl Ester |
| tert-butyl N-(6-aminohexyl)carbamate |
| Carbamic acid, N-(6-aminohexyl)-, 1,1-dimethylethyl ester |
| MFCD00671489 |
| 2-Methyl-2-propanyl (6-aminohexyl)carbamate |
| N-(tert-Butoxycarbonyl)-1,6-hexanediamine |
 CAS#:124-09-4
CAS#:124-09-4 CAS#:24424-99-5
CAS#:24424-99-5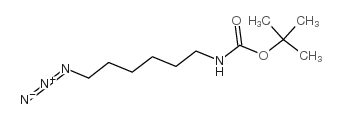 CAS#:129392-87-6
CAS#:129392-87-6 CAS#:6627-89-0
CAS#:6627-89-0 CAS#:118110-05-7
CAS#:118110-05-7 CAS#:34619-03-9
CAS#:34619-03-9![N-{6-{[(tert-butoxy)carbonyl]amino}hexyl}-2,2,2-trifluoroacetamide Structure](https://image.chemsrc.com/caspic/211/251557-12-7.png) CAS#:251557-12-7
CAS#:251557-12-7 CAS#:6404-29-1
CAS#:6404-29-1 CAS#:85535-56-4
CAS#:85535-56-4 CAS#:60-32-2
CAS#:60-32-2![(6-[(ACRIDINE-9-CARBONYL)-AMINO]-HEXYL)-CARBAMIC ACID TERT-BUTYL ESTER structure](https://image.chemsrc.com/caspic/387/259222-02-1.png) CAS#:259222-02-1
CAS#:259222-02-1 CAS#:65915-94-8
CAS#:65915-94-8![tert-butyl (6-(5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)hexyl)carbamate structure](https://image.chemsrc.com/caspic/139/153162-70-0.png) CAS#:153162-70-0
CAS#:153162-70-0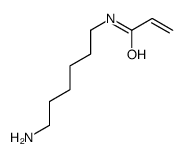 CAS#:7530-30-5
CAS#:7530-30-5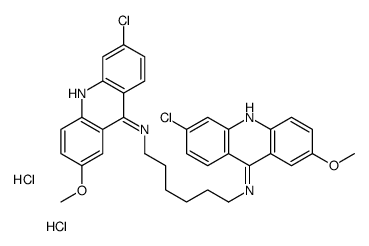 CAS#:75340-78-2
CAS#:75340-78-2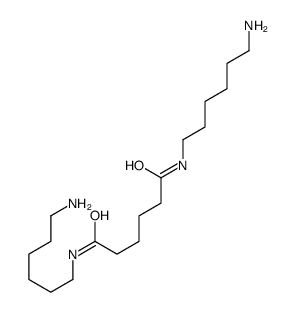 CAS#:65170-34-5
CAS#:65170-34-5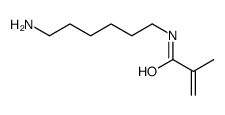 CAS#:65915-97-1
CAS#:65915-97-1
