NH2-C4-NH-Boc
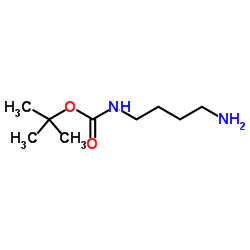
NH2-C4-NH-Boc structure
|
Common Name | NH2-C4-NH-Boc | ||
|---|---|---|---|---|
| CAS Number | 68076-36-8 | Molecular Weight | 188.267 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 292.9±23.0 °C at 760 mmHg | |
| Molecular Formula | C9H20N2O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 130.9±22.6 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
Use of NH2-C4-NH-BocNH2-C4-NH-Boc (compound 15) is a PROTAC linker, which refers to the Alkyl/ether composition. NH2-C4-NH-Boc can be used in the synthesis of a series of PROTACs. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[1]. |
| Name | tert-Butyl N-(4-aminobutyl)carbamate |
|---|---|
| Synonym | More Synonyms |
| Description | NH2-C4-NH-Boc (compound 15) is a PROTAC linker, which refers to the Alkyl/ether composition. NH2-C4-NH-Boc can be used in the synthesis of a series of PROTACs. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[1]. |
|---|---|
| Related Catalog | |
| Target |
Alkyl/ether |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 292.9±23.0 °C at 760 mmHg |
| Molecular Formula | C9H20N2O2 |
| Molecular Weight | 188.267 |
| Flash Point | 130.9±22.6 °C |
| Exact Mass | 188.152481 |
| PSA | 64.35000 |
| LogP | 1.06 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.457 |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C |
| Risk Phrases | R34 |
| Safety Phrases | S26-S36/37/39-S45 |
| RIDADR | UN 2735 8/PG 3 |
| WGK Germany | 3 |
| Hazard Class | 8.0 |
| HS Code | 2924199090 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Peptide splicing in a double-sequence analogue of trypsin inhibitor SFTI-1 substituted in the P₁ positions by peptoid monomers.
Biopolymers 104 , 206-12, (2015) Recently, we described a process of trypsin-assisted peptide splicing of analogs of trypsin inhibitor SFTI-1, that seems to be very similar to proteasome-catalyzed peptide splicing. Here, we show, for... |
|
|
Synthesis, tubulin binding, antineoplastic evaluation, and structure-activity relationship of oncodazole analogues.
J. Med. Chem. 32 , 409, (1989) In an attempt to identify a soluble oncodazole analogue that could be easily formulated, a series of substituted oncodazoles was synthesized and evaluated for tubulin binding affinity, in vitro cytoto... |
|
|
Synthesis and biological evaluation of new citrate-based siderophores as potential probes for the mechanism of iron uptake in mycobacteria.
J. Med. Chem. 45 , 2056, (2002) Several iron chelators containing alpha,beta-unsaturated hydroxamic acid motifs appended to a citric acid platform were synthesized. Mycobacterium paratuberculosis was then challenged to grow in the p... |
| 2-Methyl-2-propanyl (4-aminobutyl)carbamate |
| BOC-NH(CH2)4NH2 HCL |
| tert-butyl 4-aminobutylcarbamate |
| Carbamic acid, N-(4-aminobutyl)-, 1,1-dimethylethyl ester |
| (4-amino-butyl)-carbamic acid tert-butyl ester |
| N-(1,1-dimethylethoxycarbonyl)-1,4-butanediamine |
| BOC-DIAMINOBUTANE HCL |
| N-Boc-1,4-butanediamine |
| N-Boc-1,4-butandiamine |
| N-T-BOC-BUTANDIAMINE HCL |
| (4-aminobutyl)carbamic acid 1,1-dimethylethyl ester |
| BUTTPARK 121 6-09 |
| RARECHEM AR PA 0023 |
| tert-ButylN-(4-aminobutyl)carbamate |
| 4-(t-butoxycarbonylamino)butylamine |
| N-BOC-1,4-DIAMINOBUTANE |
| AURORA KA-4397 |
| N-(tert-butoxycarbonyl)-1,4-diamino-butane |
| BOC-DAB HCL |
| N-tert-Butoxycarbonyl-1,4-butanediamine |
| MFCD02090802 |
| tert-butyl 4-amino butylcarbamate |
 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:110-60-1
CAS#:110-60-1![2-[[(tert-butoxycarbonyl)oxy]imino]-2-phenylacetonitrile Structure](https://image.chemsrc.com/caspic/059/73371-96-7.png) CAS#:73371-96-7
CAS#:73371-96-7 CAS#:26364-65-8
CAS#:26364-65-8 CAS#:91419-50-0
CAS#:91419-50-0 CAS#:33545-97-0
CAS#:33545-97-0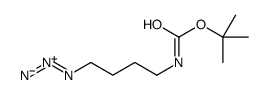 CAS#:129392-85-4
CAS#:129392-85-4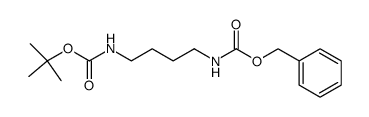 CAS#:62146-59-2
CAS#:62146-59-2 CAS#:6627-89-0
CAS#:6627-89-0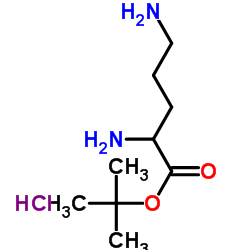 CAS#:33545-98-1
CAS#:33545-98-1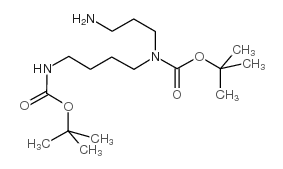 CAS#:68076-39-1
CAS#:68076-39-1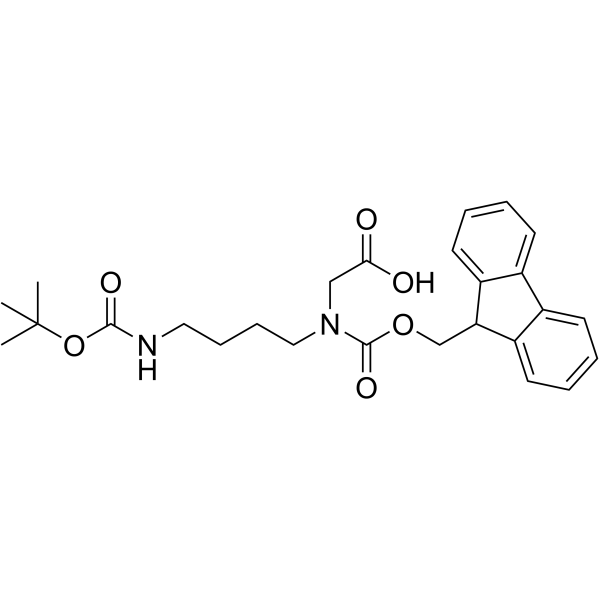 CAS#:171856-09-0
CAS#:171856-09-0![Carbamic acid, [4-(ethylamino)butyl]-, 1,1-dimethylethyl ester (9CI) structure](https://image.chemsrc.com/caspic/175/780802-42-8.png) CAS#:780802-42-8
CAS#:780802-42-8![tert-butyl N-[4-(2-cyanoethylamino)butyl]carbamate structure](https://image.chemsrc.com/caspic/163/68076-40-4.png) CAS#:68076-40-4
CAS#:68076-40-4 CAS#:99733-14-9
CAS#:99733-14-9 CAS#:62146-62-7
CAS#:62146-62-7![tert-butyl N-[4-[(2,5-dioxopyrrolidin-1-yl)oxycarbonylamino]butyl]carbamate structure](https://image.chemsrc.com/caspic/457/404004-37-1.png) CAS#:404004-37-1
CAS#:404004-37-1
